| Learning Aims: | |||||||||||||||||||||
|
|||||||||||||||||||||
| Materials: | |||||||||||||||||||||
|
|||||||||||||||||||||
| Suggestions for use: | |||||||||||||||||||||
|
Activity 1 engages students into the field of X-ray images and their interpretations. Let the students answer the given questions and discuss their answers with the whole class. Act as facilitator of the discussion. In activity 2 students explore the process of attenuation. They have to design experiments to answer two research questions: Experiment A. In order to demonstrate how the degree of absorption depends on the material, 2 mm plates of Plexiglas, aluminium, steel and lead can be used. Exemplary data: Co-60 gamma radiation, measurement time: t = 10 s
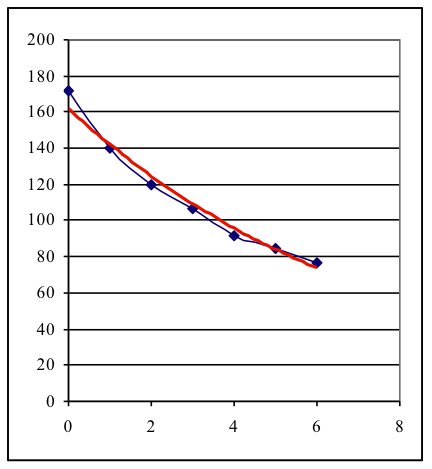
The measurements show that substances having a higher atomic number (“heavy elements”) are better able to attenuate gamma radiation than substances with a lower number. It can be seen that lead shields gamma radiation the best but it is able to penetrate even a 2 mm layer of lead but only a small part of the radiation is attenuated. Experiment B. The thicker the material, the more the gamma radiation is attenuated. This effect may be demonstrated by using lead plates, since the thickness of lead needed for a half-value layer can be easily achieved in an experiment. Exemplary data: Co-60 gamma radiation; t = 10 s The resulting graph (blue: measurement, red: function-fit) shows the exponential decrease.
The HVL for lead is approximately 12 mm. Activity 3 explains the law of attenuation and gives definition of the concept Half Value Layer. Activity 4 encourages students to apply the learned concepts to human tissues. Calculated HVLs for soft tissue and bone at 30 and 60 keV are:
|
|||||||||||||||||||||
| Possible questions: | |||||||||||||||||||||
|