Engaging questions:
- What happens to the light when it passes through the solution of the coloured substance?
- How do the so-called tinted windows in cars work?
- How does the floor under the stained-glass window look-like?
- Does the light intensity change after passing through a substance?
- Do the colour of the light influence the change in its intensity when passing through the sample?
- How to choose the colours of light, so as for the sample of a particular colours, the changes in the light intensity would be the greatest?
Chemicals:
- CuSO4 – solution 0.5 mol/dm3 ,
Equipment:
- colorimeter
- cuvettes – 5 pieces
- pipettes 10 ml – 2 pieces
- beakers 25 ml – 7 pieces
Description of the Activity :
- Prepare a series of aqueous CuSO4 solutions in range 0.1 - 0.4 mol/dm3 by diluting the standard solution. A dilution scheme:
|
Solution [mol/dm3] |
Preparation |
|
1. 0.5 |
standard |
|
2. 0.4 |
8 cm3 of solution 1 + 2 cm3 of water |
|
3. 0.3 |
6 cm3 of solution 1 + 4 cm3 of water |
|
4. 0.2 |
4 cm3 of solution 1 + 6 cm3 of water |
|
5. 0.1 |
2 cm3 of solution 1 + 8 cm3 of water |
|
6. 0 |
water |
- Perform colorimetric measurements for all the samples for different colours.
- Plot the voltage dependence on concentration.
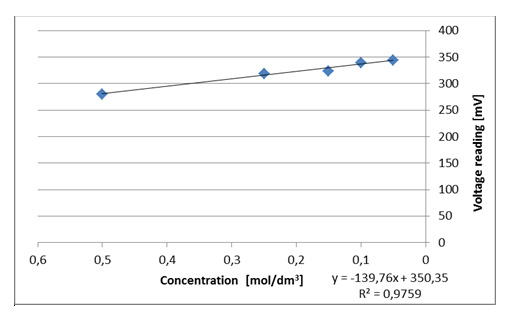
Figure III.8. An example of the dependence of the recorded voltage on the concentration of copper(II) cations for the red colour.
Discussion:
- What light colours should be chosen for the measurements?
- What is the nature of SEM dependence on concentration?
- How could you get similar result, without changing the concentration of the solution?
- You already know the voltage dependence on the sample concentration. Could you use this data to determine the CuSO4 concentration in an unknown sample?
- Why do we perform measurements for pure water?
- Does the test sample concentration have to be in the range 0.1 - 0.5 mol/dm3?
It is possible to modify the colorimeter described in the Activity III.1, for measuring the dependence of the light intensity that passes through the sample on the optical path length. To do so, the cuvette holder should be prepared in a way that enables placing in it from 1 to 5 cuvettes.