Engaging questions:
- Why are the solutions of iron(III), iron(II), copper(II), cobalt(II) coloured, and the solutions of e.g. sodium and potassium ions not?
- What determines the colour of the coordination compounds?
Chemicals:
CoCl2×6H2O CAS: [7791-13-1]
HCl 12 mol/dm3 CAS: [7647-01-0]
ethanol 95% CAS: [64-17-5]
Equipment: 5 test-tubes, technical scales (giving results in two decimal places), a spatula, measuring cylinder.
Description of the Activity :
On the technical scales, students weigh out 0.1 g CoCl2×6H2O for each of the test-tubes and for each of them they add 10 drops of concentrated hydrochloric acid. Then, they dilute obtained solutions with a solvent of a composition presented in the Table 1.
Table 1. The composition of a solvent
|
Number of the test-tube |
Solvent [% vol.] |
|
1 |
100 % of deionised water |
|
2 |
50% of water - 50% of ethanol |
|
3 |
20% of water - 80% of ethanol |
|
4 |
5% of water - 95% of ethanol |
|
5 |
100% ethanol |
Discussion:
- What are the differences in the solution colours in the test-tubes?
- Is there any regularity in the change of colour?
- What ions are present in the test-tube 1 and 5?
- What is the structure of the formed coordination compounds?
- In Figure III.9 the spectra of solutions number 1 and 5 are presented:
- What determines the position of the peak in the spectrum?
- What determines the height of the peak in the spectrum?
- How will the spectra for the test-tubes number 2-4 look like?
- Are Sc and Zn included to transition metals?
- Why +II is the typical degree of oxidation for many d-block elements?
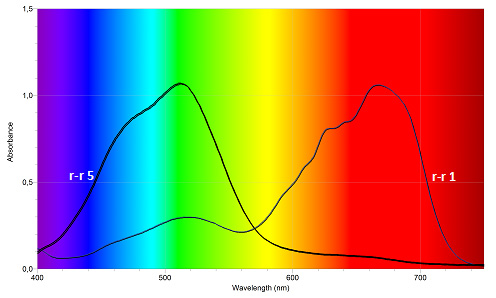
Figure III.9. UV-Vis spectrum for the samples 1 to 5. [1]
[1] Interestingly, a local maximum for the solution 1 spectrum at about 520 nm comes from the water of hydration.