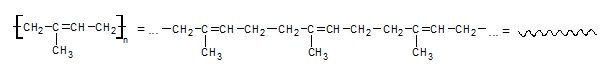| Learning Aims: |
|
| Materials: |
|
| Suggestions for use: |
|
Procedure:
Discussion: Synthetics polymers are substances which consist of long chains of big molecules – macromolecules. Their structure is similar to chain. The macromolecules are composed of a large number of smaller periodically repetitive units like segments of chain.
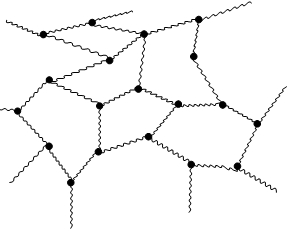
Linear polymer White glue is a mixture of polymer and water. Molecules of the polymer are stacked like small pieces of spaghetti. Tangled molecules give to the white glue sticky character but it is not liquid. If we put the white glue on air, the water from the glue is evaporating from the polymer and polymer chains are stacked to the surfaces and connect them together. If we add a Borax, which contains borate ions, the ions can form a connection between the chainsof the polymer molecules and three-dimensional network is formed. As a consequence, the properties are changed and the Gluep is more solid than the original white glue (like in the case if you overcook the spaghetti).Of course, Gluep is also less sticky and elastic than the original white glue.
You know that white glue is linear non-cross-linked polymer. What happened after addition of borax? The cross-linked polymer has been formed with three-dimensional polymer network. Chains has been prolonged. |
| Possible questions: |
The Gluep is more solid than the original white glue (like in the case if you overcook the spaghetti).Of course, Gluep is also less sticky and elastic than the original white glue.
White glue is a mixture of polymer and water. Molecules of the polymer are stacked like small pieces of spaghetti. Tangled molecules give to the white glue sticky character but it is not liquid. If we put the white glue on air, the water from the glue is evaporating from the polymer and polymer chains are stacked to the surfaces and connect them together. |